

Chamaebatia foliiolosa Benth., Sierra Nevada mountains.
Purshia tridentata (Pursh.) DC in California
Rosaceae Actonorhizal Species |
||
| Cercocarpus ledifolius Nutt. - Curl-leaf mountain mahogany C. intricatus S. Wats -littleleaf mountain mahogany C. montanus Raf.- Alderleaf mountain mahogany C. betuloides Torrey & A. Gray- Birch Leaf Mountain Mahogany C. minutiflorus Abrams - Smooth mountain mahogany |
C. traskiae Eastw. - Catalina Island mountain mahogany Chamaebatia foliolosa Benth. - mountain misery C. australis (Brandeg.) - Mountain misery Dryas drummondii Richards. ex Hook., - Dryas P. glandulosa Curran - Desert bitterbrush P. mexicana (D. Don) Henrickson - Mexican cliffrose (formerly Cowania) |
Purshia stansburyana (Torr.) Henrickson - Stansbury cliffrose P. tridentata (Pursh.) DC. - Antelope bitterbrush P. ericifolia (Torr. ex Gray) Henrickson - heath cliffrose P. subintegra (Kearney) Henrickson |
(Ecology of the Rosaceae symbioses)
The Rosaceae has about 122 genera and 3000 species (Heywood, 1993). They are distributed worldwide, especially in north temperate regions. Four genera are considered to be actinorhizal. Three of the four (Cercocarpus, Chamaebatia and Purshia are limited to the Western North America and the fourth, Dryas, is circumboreal but apparently is only nodulated in North America.
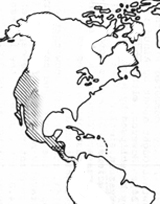
A.
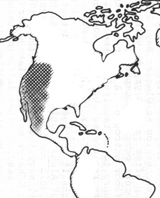
B.
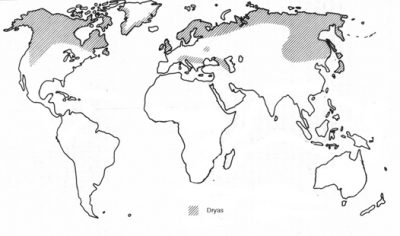 C.
C.
Fig. 3. Native distribution of Cercocarpus (A), Purshia (B) and Dryas (C). Redrawn from Silvester (1977).
Traditionally, the Rosaceae has been subdivided into four subfamilies; the Rosoideae, the Spiraeoideae, the Maloideae, and the Amygdaloideae on the basis of fruit type (Schulze-Menz, 1964). The first rbcL phylogeny constructed for the Rosaceae found that the traditional subfamilies were not natural. Instead, clades corresponded to base chromosome number rather than fruit type (Morgan, et al., 1994).
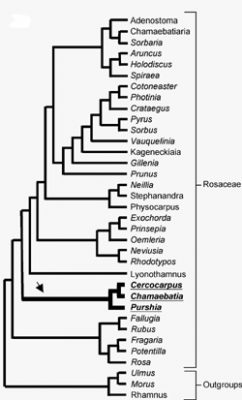
Later phylogenetic studies using other molecular markers, have shown that the four actinorhizal genera of the Rosaceae are together in a separate clade (Fig. 2). These genera include Cercocarpus HBK (6 to 10 species restricted to southwestern North America), Purshia (eight species also restricted to southwestern North America), Chamaebatia (2 species found in California), and Dryas (2 or 3 species found circumpolar in alpine and artic habitats; Fig. 3) (Evans, et al., 2000; Morgan, et al., 1994; Potter, 1997; Potter et al., 2002). The previously named Cowania was combined with Purshia under the name Purshia (Henrickson, 1986). This orientation suggests that the nodulation trait evolved once as the family diverged, or that it was present in the common ancestor of the family and was lost twice in its diversification.
Nodulation in the field of the rosaceous actinorhizal plants is sporadic (Klemmedson, 1979). Nodulation rates of between 8.3% to 32.2% have been reported for field plants of Cercocarpus, Cowania and Purshia (Nelson, 1983). Some species of Dryas have not been observed to nodulate (Kohls, et al., 1994). Both D. octapetala and D. integrifolia were reported to nodulate in older literature but the observations are in need of verification (Baker and Schwintzer, 1990). A putative hybrid between D. drummondii and D. integrifolia found in Glacier Bay National Park, D. drummondii, var. eglandulosa, apparently does not nodulate even when deliberately inoculated in the greenhouse (Kohls, et al., 1994). When Dryas or other actinorhizal rosaceous plants are inoculated in the greenhouse with either soil or crushed nodules, nodules develop beginning six to eight weeks after inoculation. This slow development contrasts with the two to three weeks normally required for nodules to appear on inoculated Alnus or Myrica.