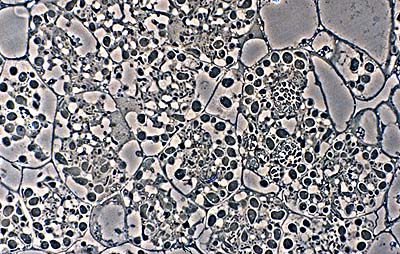
Vesicle clusters in plant cells (in this example, alder) form the starting material for isolation attempts. (R. H. Berg)
Frankia is difficult to isolate directly from soil, so most strains originate from root nodules. Two factors limit success, one is that Frankia strains grow slowly, and the other is that fast-growing contaminants are common. To minimize the second problem, nodules are disinfected with dilute sodium hypochlorite and then peeled. Vesicle clusters can be separated from plant tissue by differential screening (Benson, 1982) or density centrifugation (Baker & O'Keefe, 1984). Clusters are best pour-plated on a variety of media and followed microscopically until they begin to grow over a period of ten days to three weeks. Monitoring the outgrowth of hyphae microscopically improves the chances of obtaining a monoculture. Contaminants are spatially removed from the slower-growing Frankia colony.
The medium used in isolating new Frankia strains is important but universal, or selective, media have not been reported. Effective media range from defined propionate media (DPM; Baker, 1984), to the complex QMod medium of Lalonde and Calvert (Lalonde, 1979); we have had success using R2A medium of Reasoner and Geldreich (1985) which uses pyruvate as a carbon source. Antifungal agents, like cycloheximide or nystatin, can minimize fungal contamination. Other additions include root-lipid extracts to stimulate growth during isolation (Quispel, 1983), although we have not found this necessary.
Growth Characteristics
Frankia strains are heterotrophic aerobes having generation times of 15 or more hours. Some strains, like the Casuarina infective strain HFPCcI3, prefer lower O2 levels than others and grow poorly in vigorously aerated media, particularly at low cell density. This is in contrast to other strains such as HFPCpI1 which grows well to high density in liquid medium with shaking. As a consequence of their filamentous morphology, the growth kinetics of Frankia strains generally consist of a stationary phase after transfer, followed by a short 'exponential' phase, and then by a slower quasi-linear increase in biomass over time (Huang and Benson, 2012). Problems typical of growing other filamentous organisms apply to Frankia strains. Care must be taken to avoid nutrient and waste gradients across mycelia and a flocs or pellet formation should be avoided by frequent homogenization (Benson and Schultz,1990).
Growth and maintenance media range from simple basal salts media containing pyruvate or propionate, to complex media containing, in addition to basal salts, yeast extract, bovine serum albumin, malt extract, beef extract, NZ Amine A, casamino acids, vitamin supplements, or Tweens (Lechevalier, 1990).
Virtually all Frankia strains isolated require no growth factors, and thus grow well in defined minimal medium (FDM) . Some are inhibited by undefined media additives such as yeast extract (Lechevalier, 1990). Another general medium for Frankia is defined propionate minimal medium (DPM) medium of Baker and O'Keefe (Baker, 1984), or BAP medium (Murry et al., 1984). We use a modified FDM medium for growing CcI3 on plates called CB-CcI3.
Most strains are grown and maintained in liquid culture, and generally grow slowly on solid media (Bassi and Benson, 2007). Colonies form from spores or mycelial fragments after about seven to ten days, or longer, after plating, under the best of conditions. Some types of agar may be inhibitory, while gellan gum (Phytogel) has proven to be a suitable substitute, especially for spore germination studies.
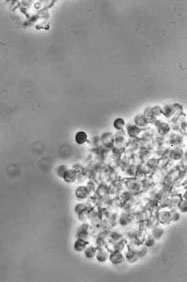
Alnus glutinosa vesicle clusters isolated from disrupted alder nodules (Phase contrast) (D. Benson)
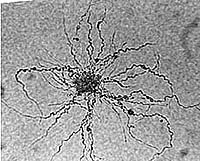
Alder Frankia growing from a vesicle cluster on agar; viewed with LM (D. Benson)

AFrankia alni CpI1 grown shaking (left) and shaking with Carbopol (right). Note pellets formed versus dispersed growth (Bourret & Harriott, 1992)lder Frankia growing from a vesicle cluster on agar; viewed with LM (D. Benson)